Introduction: BCMA-directed CAR-T and Bispecific T-cell engager (BiTE) therapies have shown remarkable overall response rates in heavily pretreated patients with relapsed/refractory multiple myeloma (RRMM). This has led to the approval of idecabtagene vicleucel, ciltacabtagene autoleucel and teclistamab by the FDA, EMA and other regulatory agencies. We performed a global survey on accessibility, practice patterns, and toxicity management of commercially available CAR-T and BiTE therapies.
Methods: An online survey was completed by clinicians attending the International Myeloma Society's Immune Effector Cell Therapies in Multiple Myeloma Workshop, in-person or virtually, made available for 30 days after the meeting held in March 2023, Boston, MA.
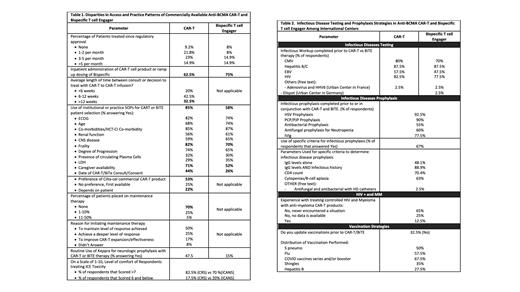
Results: 87 clinicians from 16 countries completed the survey, most practicing in an academic practice (86%) and in an urban environment (89%). Sixty-four percent of the respondents practice in North America followed by Europe (25.3%) including Germany, United Kingdom, Ireland, Italy, Netherlands, Spain, France, Greece, and Cyprus, and 6.9% from Asia and 3.4% from Australia. Seventy-seven percent reported CAR-T cell therapy was approved in their country with 44.8% treating 1-5 patients and 14.9% treating >5 patients per month; 67% reported BiTE therapy was approved in their country with 14.9% treating >5 patients per month. Of evaluable responses (n = 40), 62% administered CAR-T therapy inpatient and 15% administered CAR-T therapy outpatient ≥ 50% of the time. Length of time between decision to treat with CAR-T therapy to infusion was 6-12 weeks for 42% of responders and <6 weeks for only 20%. Most (75%) reported inpatient administration of BiTE therapy step-up dosing; 17.5% reported outpatient step-up dosing administered ≥ 50% of the time. Use of institutional or practice SOPs for CAR-T and BiTE therapy patient selection was reported by 85% and 58%, respectively, with the factors influencing SOP outlined in Table 1. Of available CAR-T therapies, 53% prefer cilta-cel and 23% reported CAR-T therapy preference depended most on patients' co-morbidities/frailty (89%), timing of next available apheresis (67%), age (56%), CNS disease (44%) and other factors. Maintenance therapy after CAR-T therapy was reportedly used by 25% and 5% of respondents in 1-10% and 11-50% of their patients, respectively, to “maintain level of response achieved” (50%) or “achieve a deeper level of response” (25%). About half (43%) of the respondents reported using an SOP or particular criteria for secondary Hemophagocytic Lympho-Histiocytosis/Macrophage Activation Syndrome/CAR-related-HLH (sHLH/MAS/CarHLH) management that varied between the use of ASTCT Working Group IEC associated HLH-like syndrome Criteria (47%) (Hines 2023), CARTOX HLH Criteria (24%) (Neelapu 2017) and HLH-2004 criteria (6%).
Tocilizumab was used as cytokine release syndrome (CRS) prophylaxis only by one responder. Keppra neurologic prophylaxis was used by 47.5% and 15% of responders for CAR-T and BiTE therapy, respectively. Delayed neurotoxicity with CAR-T was reported in 1-5% of patients by 57.5% of responders and 6-10% of patients by 15% of responders; most common manifestations were other movement/neuromuscular disorder (62%), nerve palsy (55%), and Parkinsonism (45%).
Infectious prophylaxis was used to protect against HSV (92.5%), PJP (90%), bacterial pathogens (55%), fungal pathogens (60%), and IVIG (77.5%). However, criteria for infectious prophylaxis use varies widely incorporating IgG levels, infectious history, CD4+ count, B-cell aplasia and cytopenias (48.1 - 88.9%). Most respondents (46.3 - 86.5%) reported completing an infectious work-up including CMV, EBV, Hep B/C, and HIV ( Table 2).
Conclusions: Trends of CAR-T and BiTE therapy use and patterns of concurrent prophylactic and supportive care in MM vary widely among academic centers across the globe, demonstrating a need for further understanding of toxicity pathogenesis, toxicity management and consistent guidelines. This survey also demonstrates the great unmet need for access beyond urban centers among the international myeloma community. Prospective studies and claims data analysis should be utilized to evaluate the longitudinal cost of care, quality of life, duration of response and management of infectious disease, neurological or related complications of CAR-T and bispecific TCE therapies.
Disclosures
Cohen:Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; BMS/Celgene: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy; Ichnos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Arcellx: Consultancy. Moreau:GSK: Honoraria, Other: Advisory Board; janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards. Rodríguez Otero:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Roche: Consultancy; Regeneron: Other: Honoraria for lectures; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Amgen: Other: Honoraria for lectures; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures. Mateos:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; University of Salamanca/Gerencia Regional de Salud de Castilla y León: Current Employment; Regeneron: Honoraria; Amgen: Honoraria; Takeda: Honoraria; BMS-Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Avigan:Karyopharm Therapeutics: Consultancy, Other: Advisory role; Partner Therapeutics: Consultancy, Other: Advisory board; Juno Therapeutics: Consultancy, Other: Advisory role; Legend Biotech: Consultancy, Other: Advisory role; Kite/Gilead: Consultancy, Other: Advisory role, Research Funding; Kowa Pharmaceutical: Consultancy, Other: Advisory board; Sanofi: Consultancy, Other: Advisory board; Chugai Pharma: Consultancy, Other: Advisory role; Paraxel: Current Employment; Celgene: Consultancy, Other: Advisory role, Research Funding; Janssen: Consultancy, Other: Advisory board; Aviv Med Tech: Consultancy, Other: Advisory board; Bristol-Myers Squibb: Consultancy, Other: Advisory board; Takeda: Consultancy, Other: Advisory role; Pharmacyclics: Research Funding; Kite, a Gilead Company: Research Funding. Sperling:Roche: Consultancy; Novartis: Consultancy. Nadeem:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; GPCR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Fernández de Larrea:GSK: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding. Berdeja:AbbVie: Research Funding; Ichnos Sciences: Research Funding; Takeda: Consultancy, Research Funding; Roche: Consultancy; C4 Therapeutics: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Karyopharm: Research Funding; Teva: Research Funding; Fate Therapeutics: Research Funding; CARsgen: Research Funding; Celgene: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy, Research Funding; Sanofi: Research Funding; Acetylon: Research Funding; Incyte: Research Funding; Poseida: Research Funding; Novartis: Research Funding; 2seventy bio: Consultancy, Research Funding; Genentech: Research Funding; Lilly: Research Funding; Legend Biotech: Consultancy; Amgen: Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Kite Pharma: Consultancy; GSK: Research Funding; EMD Serono: Research Funding; Celularity: Research Funding; Cartesian: Research Funding. Sonneveld:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Gay:AbbVie: Honoraria, Other: Advisory board; Bristol Myers Squibb/Celgene: Honoraria, Other: Advisory board; Oncopeptides: Other: Advisory board; Sanofi: Honoraria, Other: Advisory board; Pfizer: Honoraria, Other: Advisory board; Roche: Other: Advisory board; Takeda: Honoraria, Other: Advisory board; Janssen: Honoraria, Other: Advisory board; Amgen: Honoraria, Other: Advisory board; GlaxoSmithKline: Honoraria, Other: Advisory board. Krishnan:Adaptive Biotechnologies Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline, Regeneron Pharmaceuticals Inc, Sanofi Genzyme: Other: Consulting Agreements; Sutro Biopharma: Other: Advisory Committee; Bristol-Myers Squibb Company: Other: Stock Options/Ownership-Public Company; Janssen Biotech Inc: Other: Contracted Research; Amgen Inc, Bristol-Myers Squibb Company, Takeda Pharmaceuticals USA Inc: Other: Speakers Bureau. Usmani:EdoPharma: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; Moderna: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Array Biopharma: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Anderson:Window, Starton: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; NextRNA: Current equity holder in private company; Dynamic Cell Therapies: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Oncopep: Current equity holder in private company, Current holder of stock options in a privately-held company; C4 Therapeutics, Raqia, NextRNA,Dynamic Cell Therapy: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Pfizer, Janssen, Astrazeneca, Daewoong, Amgen, Starton, OncoPep, Precision Biosciences, Window Therapeutics, Mana Therapeutics: Membership on an entity's Board of Directors or advisory committees. Jagannath:Regeneron: Consultancy; Caribou: Consultancy; Janssen: Consultancy; Bristol Myers Squibb: Consultancy; Legend Biotech: Consultancy; Karyopharm: Consultancy; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees. Einsele:Novartis: Honoraria, Other: Consulting or advisory role, Travel support; Sanofi: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; GlaxoSmithKline: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Takeda: Honoraria, Other: Consulting or advisory role, Travel support; Amgen: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Janssen: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding. Midha:Abbvie: Current equity holder in publicly-traded company; Pfizer: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal